
November 1, 2022
Valisure Detects Benzene in Dry Shampoo

November 4, 2021
Valisure Detects Benzene in Body Spray

May 25, 2021
Valisure Detects Benzene in Sunscreen

March 24, 2021
Valisure Detects Benzene in Hand Sanitizer

March 2, 2020
Valisure Detects NDMA in Metformin

September 13, 2019
Valisure Detects NDMA in Ranitidine

June 13, 2019
Valisure Detects DMF in Valsartan

Responsible Disposal of Contaminated Products

March 7, 2024
A Track Record of Action: Valisure’s FDA Citizen Petitions Driving Industry-Wide Change

March 6, 2024
FDA Citizen Petition #8: Benzene in Benzoyl Peroxide Products

November 1, 2022
FDA Citizen Petition #7: Benzene in Dry Shampoo Products

November 4, 2021
FDA Citizen Petition #6: Benzene in Body Spray Products

May 25, 2021
FDA Citizen Petition #5: Benzene in Sunscreen Products

March 24, 2021
FDA Citizen Petition #4: Benzene in Hand Sanitizer Products

March 2, 2020
FDA Citizen Petition #3: NDMA Carcinogen in Metformin

September 13, 2019
FDA Citizen Petition #2: NDMA Carcinogen in Ranitidine (Zantac) & Nizatidine (Axid)

June 13, 2019
FDA Citizen Petition #1: DMF Carcinogen in Valsartan

June 20, 2025
New Study Raises Alarms About Generic ADHD Medications
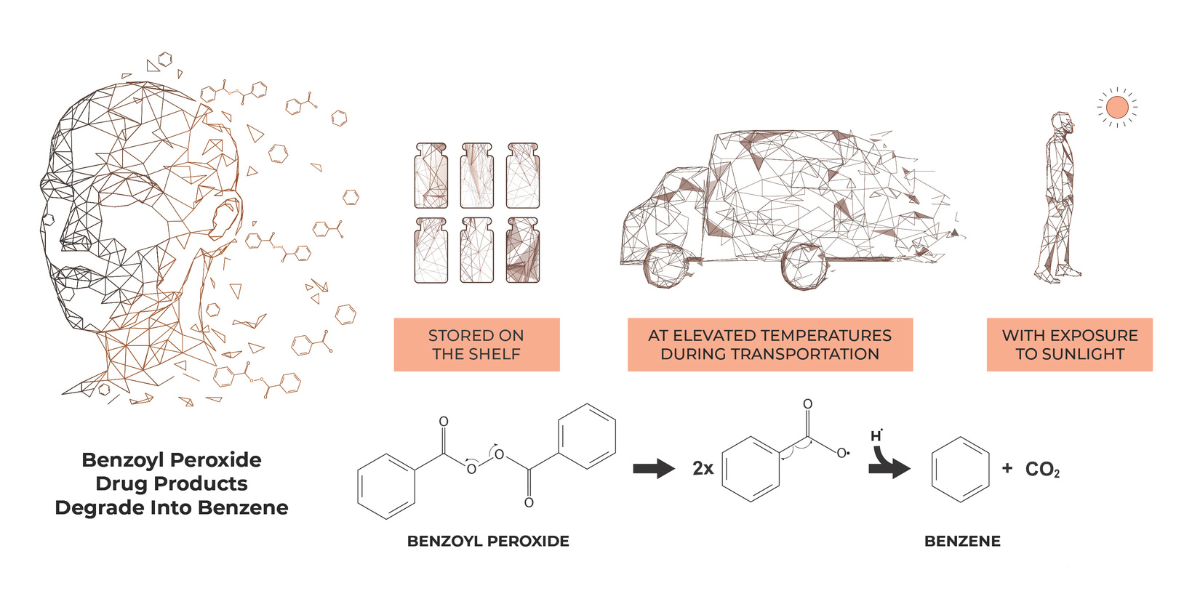
October 7, 2024
New Valisure Scientific Paper on Benzene in Benzoyl Peroxide Products

March 14, 2024
Environmental Health Perspectives Publishes Study on Benzene Formation in Benzoyl Peroxide Products

October 31, 2023
AJMC: Safety vs Price in the Generic Drug Market: Metformin

April 26, 2023
Valisure Presentation at Stanford Medicine Grand Rounds

October 10, 2022
JAPhA: A Data-Driven Quality-Score System

June 3, 2022
Journal of Pharmaceutical Innovation: FDA Approaches in Monitoring Drug Quality

April 18, 2022
Circulation: Price and Quality in the Generic Pharmaceutical Market

April 15, 2022
JDD: Dermatologists’ Responses to Benzene Being Reported as a Contaminant in Sunscreen: A Cross-Sectional Analysis

November 2, 2025
Valisure Presents at APHA 2025 Annual Meeting

October 18, 2025
Valisure Participates in the 2025 HCMA Annual Patient Meeting

October 12, 2025
Valisure Presents at DOC 2025: The Science of Longevity

June 26, 2025
Valisure Joins HCMA Webinar on Generic Drug Quality Concerns

June 25, 2025
Valisure Joins MedShadow Webinar on Generic Drug Safety

April 9, 2025
Valisure Joins HCMA Legislative Briefing to Advance Drug Quality Reform

September 25, 2024
AHRMM24 DoD-led Pharmaceutical Quality Assessment

September 19, 2024
Fireside Chat: National Security & the Pharmaceutical Supply Chain

September 13, 2024
Valisure Presents Initial Findings from Department of Defense Drug Quality Study

May 26, 2021
Dermatology Times | Valisure Highlights Controversy of Benzene Contamination in Sunscreen Products

May 26, 2021
HBW Insight | Valisure’s Benzene Testing Finds Problems With OTC Sunscreens, Cosmetic Sun Care Products

March 29, 2021
CBS News | Study Finds Carcinogen Above FDA Limit in Several Hand Sanitizer Brands

February 1, 2021
Fierce Pharma | Valisure links 'unstable' Zantac and its ilk to carcinogen buildup, cancer risk

June 3, 2020
Fierce Pharma | Are Metformin Recalls Just The Start?

May 7, 2020
Forbes | Safeguarding And Streamlining Pharma Supply Chain: Time For Mandating Certified Drugs

April 1, 2020
The New York Times | Zantac Products Should Not Be Sold or Used, F.D.A. Warns, Citing Cancer Danger

March 2, 2020
Reuters | Online Pharmacy Valisure Says Tests Show Carcinogen In Diabetes Drug Metformin

March 2, 2020
Bloomberg | Carcinogen Found by Pharmacy in Popular U.S. Diabetes Drug

May 3, 2022
Valisure Named Honorable Mention in Category of Fast Company’s 2022 World Changing Ideas Awards

April 12, 2022
David Light Awarded Junior Achievement's Entrepreneur of The Year Award

March 10, 2022
Valisure First Company To Be Named By MedPage As A Healthcare Disruptor

November 1, 2019
U.S. Senator Chris Murphy Honors Valisure With His Office’s Innovator Award

April 26, 2018
Valisure Wins Pre-Revenue Venture Of The Year In 2018 CT Entrepreneur Awards

October 20, 2025
Valisure Highlighted in Senate Aging Committee Report

April 3, 2025
Valisure Presents DoD Drug Quality Findings at State of the Science Symposium 2025

October 31, 2024
Senator Richard Blumenthal: A Champion for Medication and Consumer Product Safety

October 31, 2024
Joint Press Conference: Blumenthal & Valisure Address Benzene in Acne Products

October 28, 2024
A Shared Commitment to Drug Safety: Congresswoman Rosa DeLauro and Valisure

June 3, 2024
Valisure and FDA Leadership Meeting

April 30, 2024
Senate Finance Committee Hearing: Advancing Medication Quality for the Military

April 2, 2024
Valisure Presents at Uniformed Services University

December 29, 2023
FDA New December 2023 Guidance

























































